Lipoprotein(a) Epidemiology
Although Lp(a) is well established as a causal risk factor for cardiovascular disease, many questions still deserve further research. We are interested in the genetic and non-genetic factors which are influencing Lp(a) concentrations.
Lead
Co-Lead
Team
Genome-wide association studies (GWAS) on Lp(a)
Identifying genes and genetic variants which have an influence on Lp(a) concentrations is a way to better understand the genetic regulation of Lp(a) concentrations and the machinery which is involved in the metabolism of Lp(a). We therefore performed a meta-analysis using data from five genome-wide association studies in which all measurements of Lp(a) concentrations and apo(a) isoforms were made in our laboratory (n = 13,781). We identified 48 independent SNPs in the LPA and 1 SNP in the APOE gene region to be significantly associated with Lp(a) concentrations. Median Lp(a) values increased from 2.1 to 91.1 mg/dl with increasing number of Lp(a)-increasing alleles which underlines the strong genetic regulation of Lp(a) concentrations. Seven SNPs showed a genome-wide significant association with coronary artery disease risk (Figure 1).
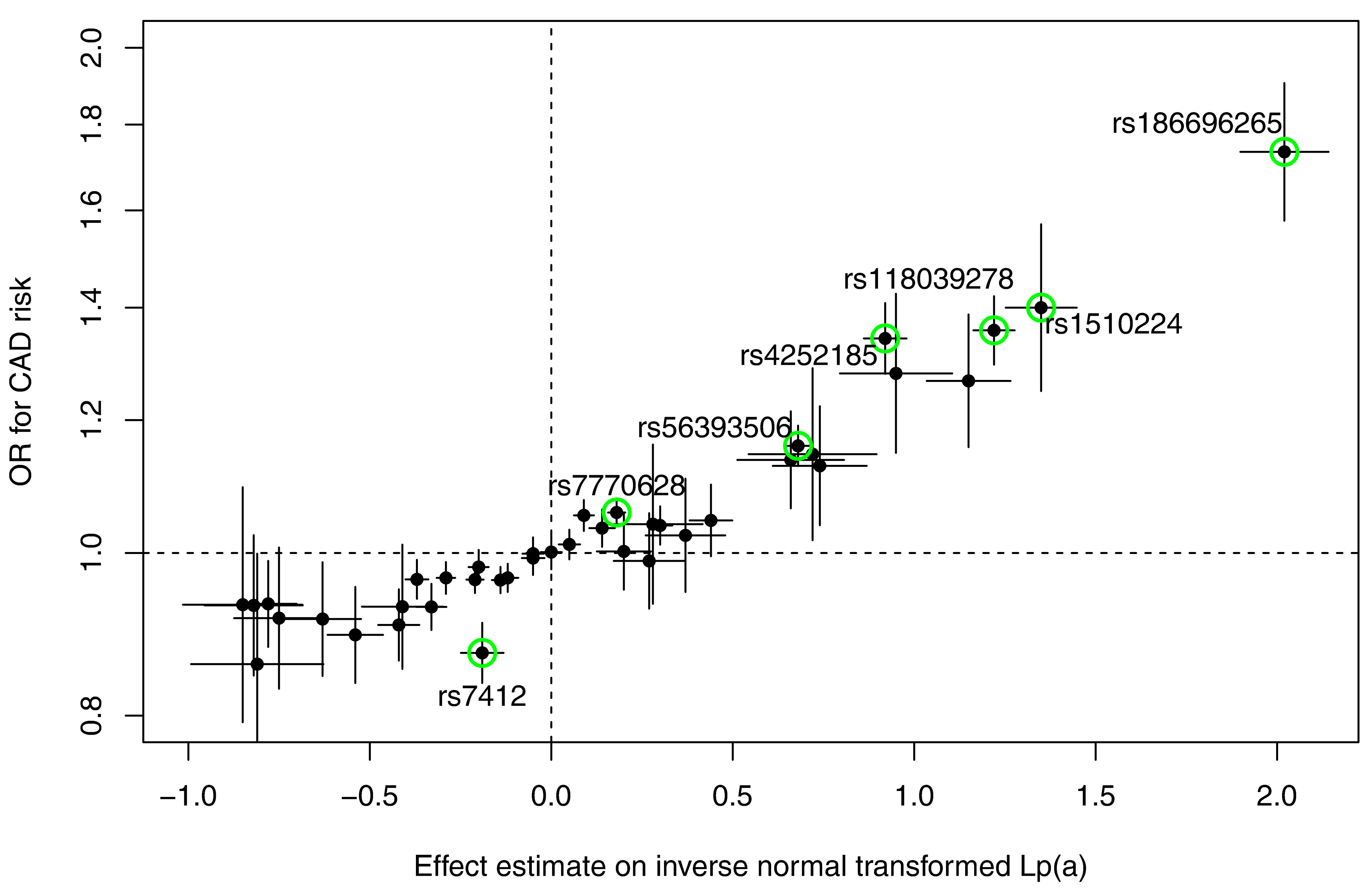
Estimation of the Lp(a)-lowering effect size for CHD reduction
Specific Lp(a)-lowering drugs are not yet available but there are promising candidates, which are currently being evaluated in clinical trials. For the planning of such trials, it is of utmost importance to estimate the required Lp(a)-lowering in order to improve clinical outcomes effectively. Using data from five studies including almost 14,000 participants, we estimated that Lp(a) would have to be reduced by 66 mg/dL in order to achieve the same effect on cardiovascular risk reduction as reducing LDL-cholesterol by 1 mmol/L by means of statins. This would correspond to a risk reduction of 45% over a lifetime or 22% in the short term (Lamina & Kronenberg JAMA Cardiol 2019). This estimation was made possible by applying a method called Mendelian randomisation. With this special statistical technique, it is possible to derive the causal relationship between a risk factor, such as Lp(a), and a disease for which genetic variants are known that regulate Lp(a) concentrations.
Lp(a) and cardiovascular disease
We investigate in various studies the association of Lp(a) with cardiovascular disease. This is been done in population-based cohorts as well as in patient cohorts and lately with data of the UK Biobank. These later studies are often done in the context with the Lp(a) Genomics program in which certain genetic variants are investigated not only in relationship with Lp(a) concentrations but also with the readout of cardiovascular endpoints.
Lp(a) and kidney disease
We investigated Lp(a) concentrations and the apo(a) size polymorphism in several patient groups with various stages of impairment of renal function and with various treatment modalities. Lp(a) concentrations are increased and are influenced by the glomerular filtration rate and the amount of proteinuria. The reason for this elevation can be an increased synthesis as this is the case for patients with nephrotic syndrome or those treated by peritoneal dialysis. In hemodialysis patients a catabolic block is the reason for this elevation. The elevated concentrations might contribute to the tremendous cardiovascular risk of tese high-risk patient groups (Figure 2).
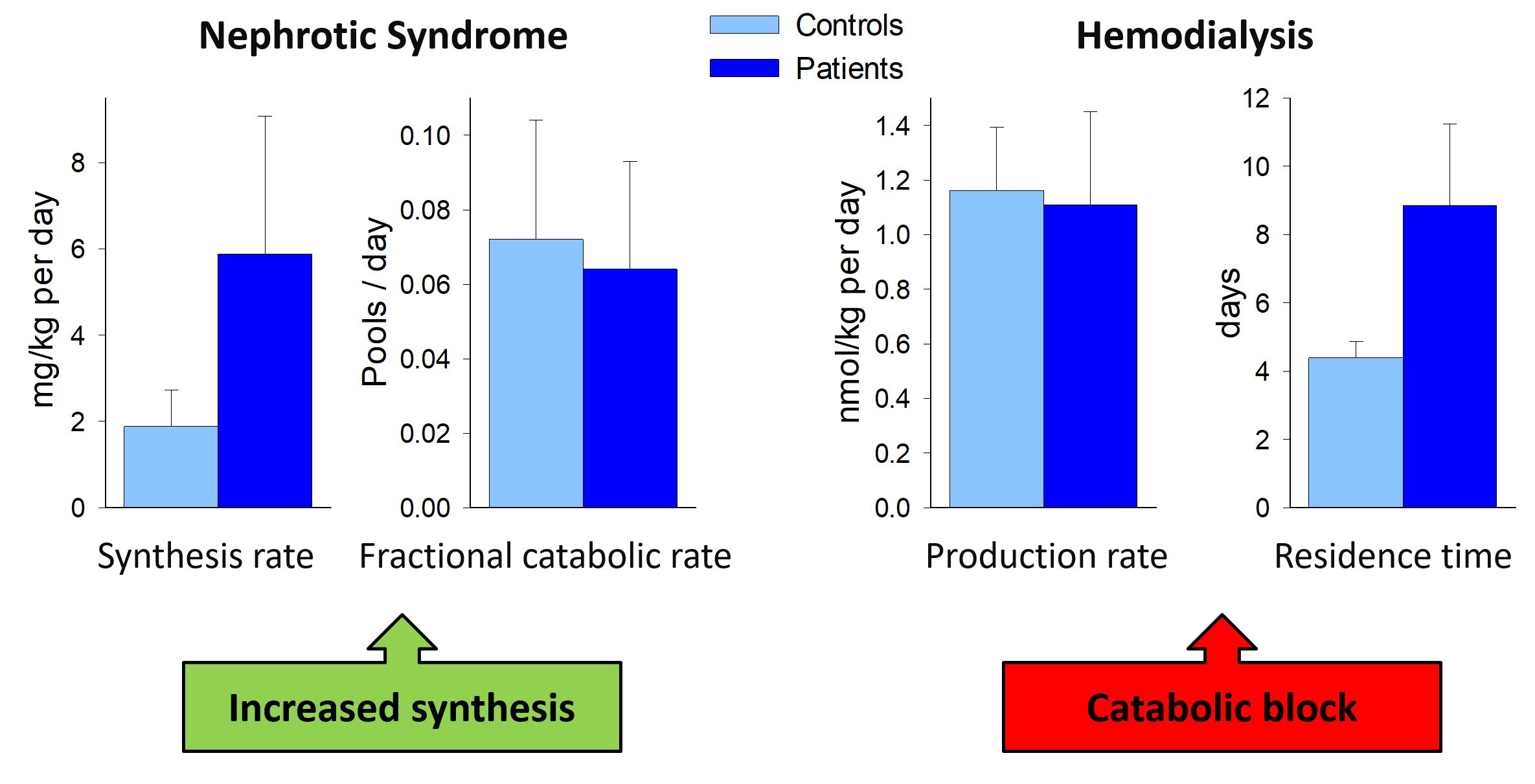
Lp(a) and peripheral arterial disease
The relevance of Lp(a) concentrations and low-molecular-weight (LMW) apo(a) phenotypes in peripheral arterial disease (PAD) has only been investigated by few studies. Therefore, we analysed this association in three independent cohorts including more than 7500 individuals and performed a Mendelian Randomization approach using instrumental variable regression. These analyses pointed to a significant association between Lp(a) concentrations, LMW apo(a) phenotypes, and one SNP within the LPA gene with symptomatic and asymptomatic PAD. This association is probably of causal nature since the genetically determined apo(a) phenotypes and the investigated SNP that influence the Lp(a) concentrations to a large extent are indeed associated with these PAD phenotypes (Laschkolnig et al.: Cardiovasc Res. 2014).
Lp(a) and SARS-CoV-2 infections
Due to the high homology of apo(a) with plasminogen, Lp(a) has repeatedly proposed as a risk factor for thromboembolic events. Since patients with SARS-CoV-2 infections have a markedly increased risk for thromboembolic events, we evaluated whether SARS-CoV-2 infections modify the risk of high Lp(a) concentrations for ischemic heart disease or thromboembolic events during the first 8.5 months follow-up of the pandemic. We used data from the UK Biobank during the first 8.5 months of the SARS-CoV-2 pandemic. The risk for ischemic heart disease increased with higher Lp(a) concentrations in both, the 435,104 population controls and the 6,937 SARS-CoV-2 positive patients. The causality of the findings was supported by a genetic risk score for Lp(a). A SARS-CoV-2 infection modified the association with a steeper increase in risk for infected patients (interaction p-value = 0.03). Most importantly, although SARS-CoV-2 positive patients had a five-times higher frequency of thromboembolic events compared to the population controls (1.53% vs. 0.31%), the risk was not influenced by Lp(a). In summary, SARS-CoV-2 infections enforce the association between high Lp(a) and ischemic heart disease but the risk for thromboembolic events is not influenced by Lp(a) (Figure 3) (Di Maio et al.: J. Intern. Med. 2022).
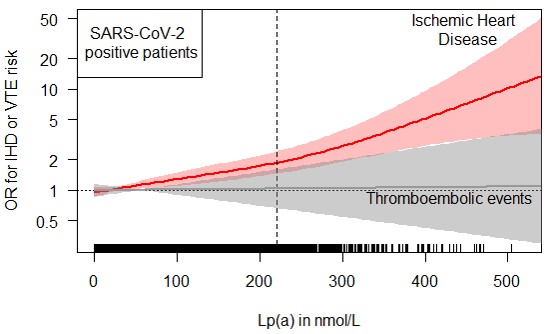
Cooperations
GCKD study, KORA Study Group, SAPHIR Study, Bruneck Study, CAVASIC Study, UK Biobank
Earlier Team Members
Salome Mack, Stephanie Stangl, Marina Haiman, Anja Laschkolnig, Barbara Rantner, Marietta Stadler, Michael Frischmann, Anita Kloss-Brandstätter, Lisa Kerschdorfer, Evi Trenkwalder, Arno Lingenhel, Doreen Dähnhardt, Margot Haun, Jamie Lee Losso,
The most important publications on this topic
Schachtl-Riess JF, Kheirkhah A, Grüneis R, Di Maio S, Schoenherr S, Streiter G, Losso JL, Paulweber B, Eckardt KU, Köttgen A, Lamina C, Kronenberg F, Coassin S, GCKD Investigators: Frequent LPA KIV-2 variants lower lipoprotein(a) concentrations and protect against coronary artery disease. J. Am. Coll. Cardiol. 78:437-449, 2021. PMID: 34325833 Journal Article
Di Maio S, Lamina C, Coassin S, Forer L, Würzner R, Schönherr S, Kronenberg F: Lipoprotein(a) and SARS-CoV-2 infections: Susceptibility to infections, ischemic heart disease and thromboembolic events. J. Intern. Med. 291:101-107, 2022. PMID: 34096654 Journal Article
Lamina C, Kronenberg F, Lp(a)-GWAS-Consortium: Estimation of the required lipoprotein(a)-lowering therapeutic effect size for reduction in coronary heart disease outcomes: A Mendelian randomization analysis. JAMA Cardiol. 4:575-579, 2019. PMID: 31017618 Journal Article
Mack S, Coassin S, Rueedi R, Yousri NA, Seppälä I, Gieger C, Schönherr S, Forer L, Erhart G, Marques-Vidal P, Ried JS, Waeber G, Bergmann S, Dähnhardt D, Stöckl A, Raitakari OT, Kähönen M, Peters A, Meitinger T, Strauch K, Kedenko L, Paulweber B, Lehtimäki T, Hunt SC, Vollenweider P, Lamina C, Kronenberg F: A genome-wide association meta-analysis on lipoprotein (a) concentrations adjusted for apolipoprotein (a) isoforms. J. Lipid Res. 58:1834-1844, 2017. PMID: 28512139 Journal Article
Coassin S, Erhart G, Weissensteiner H, Eca Guimarães de Araújo M, Lamina C, Schönherr S, Forer L, Haun M, Losso JL, Köttgen A, Schmidt K, Utermann G, Peters A, Gieger C, Strauch K, Finkenstedt A, Bale R, Zoller H, Paulweber B, Eckardt KU, Hüttenhofer A, Huber LA, Kronenberg F: A novel but frequent variant in LPA KIV-2 is associated with a pronounced Lp(a) and cardiovascular risk reduction. Eur. Heart J. 38:1823-1831, 2017. PMID: 28444229 Journal Article
Kollerits B, Drechsler C, Krane V, Lamina C, März W, Dieplinger H, Ritz E, Wanner C, Kronenberg F, German Diabetes and Dialysis Study Investigators: Lipoprotein(a) concentrations, apolipoprotein(a) isoforms and clinical endpoints in haemodialysis patients with type 2 diabetes mellitus: results from the 4D Study. Nephrol. Dial. Transplant 31:1901-1908, 2016. PMID: 26754832 Journal Article
Kronenberg F: Human genetics and the causal role of lipoprotein(a) for various diseases. Cardiovasc. Drugs Ther. 30:87-100, 2016. PMID: 26896185 Review
Laschkolnig A, Kollerits B, Lamina C, Meisinger C, Rantner B, Stadler M, Peters A, Koenig W, Stöckl A, Dähnhardt D, Böger CA, Krämer BK, Fraedrich G, Strauch K, Kronenberg F: Lipoprotein (a) concentrations, apolipoprotein (a) phenotypes, and peripheral arterial disease in three independent cohorts. Cardiovasc. Res. 103:28-36, 2014. PMID: 24760552 Journal Article
Kronenberg F: Causes and consequences of lipoprotein(a) abnormalities in kidney disease. Clin. Exp. Nephrol. 18:234-237, 2014. PMID: 24129557 Review
Kronenberg F, Utermann G: Lipoprotein(a): resurrected by genetics. J. Intern. Med. 273:Jun.30, 2013. PMID: 22998429 Review
Zabaneh D, Kumari M, Sandhu M, Wareham N, Wainwright N, Papamarkou T, Hopewell J, Clarke R, Li K, Palmen J, Talmud PJ, Kronenberg F, Lamina C, Summerer M, Paulweber B, Price J, Fowkes G, Stewart M, Drenos F, Shah S, Shah T, Casas JP, Kivimaki M, Whittaker J, Hingorani AD, Humphries SE: Meta analysis of candidate gene variants outside the LPA locus with Lp(a) plasma levels in 14,500 participants of six White European cohorts. Atherosclerosis 217:447-451, 2011. PMID: 21592478 Journal Article
Frischmann ME, Kronenberg F, Trenkwalder E, Schaefer JR, Schweer H, Dieplinger B, Koenig P, Ikewaki K, Dieplinger H: In vivo turnover study demonstrates diminished clearance of lipoprotein(a) in hemodialysis patients. Kidney Int. 71:1036-1043, 2007. PMID: 17299521 Journal Article
Kollerits B, Auinger M, Reisig V, Kästenbauer T, Lingenhel A, Irsigler K, Prager R, Kronenberg F: Lipoprotein(a) as a predictor of cardiovascular disease in a prospectively followed cohort of patients with type 1 diabetes. Diabetes Care 29:1661-1663, 2006. PMID: 16801597 Journal Article
Kronenberg F, Lingenhel A, Lhotta K, Rantner B, Kronenberg MF, König P, Thiery J, Koch M, von Eckardstein A, Dieplinger H: The apolipoprotein(a) size polymorphism is associated with nephrotic syndrome. Kidney Int. 65:606-612, 2004. PMID: 14717931 Journal Article
Other publications
Kronenberg F, Utermann G, Dieplinger H: Lipoprotein(a) in renal disease. Am. J. Kidney Dis. 27:Jan.25, 1996. PMID: 8546123 Review
Koschinsky ML, Kronenberg F: The long journey of lipoprotein(a) from cardiovascular curiosity to therapeutic target. Atherosclerosis 349:1-6, 2022. PMID: 35606069 Review
Grüneis R, Weissensteiner H, Lamina C, Schönherr S, Forer L, Di Maio S, Streiter G, Peters A, Gieger C, Kronenberg F, Coassin S: The kringle IV type 2 domain variant 4925G>A causes the elusive association signal of the LPA pentanucleotide repeat. J. Lipid Res. 63:100306, 2022. PMID: 36309064 Journal Article
Coassin S, Kronenberg F: Lipoprotein(a) beyond the kringle IV repeat polymorphism: The complexity of genetic variation in the LPA gene. Atherosclerosis 349:17-35, 2022. PMID: 35606073 Review
Grüneis R, Lamina C, Di Maio S, Schönherr S, Zoescher P, Forer L, Streiter G, Peters A, Gieger C, Köttgen A, Kronenberg F, Coassin S: The effect of LPA Thr3888Pro on lipoprotein(a) and coronary artery disease is modified by the LPA KIV-2 variant 4925G>A. Atherosclerosis 349:151-159, 2022. PMID: 35534298 Journal Article
Raitakari O, Kivelä A, Pahkala K, Rovio S, Mykkänen J, Ahola-Olli A, Loo BM, Lyytikäinen LP, Lehtimäki T, Kähönen M, Juonala M, Rönnemaa T, Lamina C, Kronenberg F, Viikari J: Long-term tracking and population characteristics of lipoprotein (a) in the Cardiovascular Risk in Young Finns Study. Atherosclerosis 356:18-27, 2022. PMID: 35961208 Journal Article
van Dam-Nolen DHK, van Dijk AC, Crombag GAJC, Lucci C, Kooi ME, Hendrikse J, Nederkoorn PJ, Daemen MJAP, van der Steen AFW, Koudstaal PJ, Kronenberg F, Roeters van Lennep JE, Mulder MT, van der Lugt A: Lipoprotein(a) levels and atherosclerotic plaque characteristics in the carotid artery: The Plaque at RISK (PARISK) study. Atherosclerosis 329:22-29, 2021. PMID: 34216874 Journal Article
Zeng L, Moser S, Mirza-Schreiber N, Lamina C, Coassin S, Nelson CP, Annilo T, Franzén O, Kleber ME, Mack S, Andlauer TFM, Jiang B, Stiller B, Li L, Willenborg C, Munz M, Kessler T, Kastrati A, Laugwitz KL, Erdmann J, Moebus S, Nöthen MM, Peters A, Strauch K, Müller-Nurasyid M, Gieger C, Meitinger T, Steinhagen-Thiessen E, März W, Metspalu A, Björkegren JLM, Samani NJ, Kronenberg F, Müller-Myhsok B, Schunkert H: Cis-epistasis at the LPA locus and risk of cardiovascular diseases. Cardiovasc. Res. 118:1088-1102, 2022. PMID: 33878186 Journal Article
Kheirkhah A, Lamina C, Rantner B, Kollerits B, Stadler M, Pohlhammer J, Klein-Weigel P, Fraedrich G, Kronenberg F: Elevated levels of serum PCSK9 in male patients with symptomatic peripheral artery disease: The CAVASIC study. Atherosclerosis 316:41-47, 2021. PMID: 33302043 Journal Article
Singh SS, Rashid M, Lieverse AG, Kronenberg F, Lamina C, Mulder MT, de Rijke YB, Sijbrands EJG, van Hoek M: Lipoprotein(a) plasma levels are not associated with incident microvascular complications in type 2 diabetes mellitus. Diabetologia 63:1248-1257, 2020. PMID: 32152647 Journal Article
Yahya R, Berk K, Verhoeven A, Bos S, van der Zee L, Touw J, Erhart G, Kronenberg F, Timman R, Sijbrands E, Roeters van Lennep J, Mulder M: Statin treatment increases lipoprotein(a) levels in subjects with low molecular weight apolipoprotein(a) phenotype. Atherosclerosis 289:201-205, 2019. PMID: 31327478 Journal Article
Kronenberg F: Prediction of cardiovascular risk by Lp(a) concentrations or genetic variants within the LPA gene region. Clin. Res. Cardiol. Suppl. 14:05.Dec, 2019. PMID: 30859385 Review
Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, Schwartz GG, Olsson AG, Colhoun HM, Kronenberg F, Drechsler C, Wanner C, Mora S, Lesogor A, Tsimikas S: Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet 392:1311-1320, 2018. PMID: 30293769 Journal Article
Berk KA, Yahya R, Verhoeven AJM, Touw J, Leijten FP, van Rossum EF, Wester VL, Lips MA, Pijl H, Timman R, Erhart G, Kronenberg F, Roeters van Lennep JE, Sijbrands EJG, Mulder MT: Effect of diet-induced weight loss on lipoprotein(a) levels in obese individuals with and without type 2 diabetes. Diabetologia 60:989-997, 2017. PMID: 28386638 Journal Article
Paige E, Masconi KL, Tsimikas S, Kronenberg F, Santer P, Weger S, Willeit J, Kiechl S, Willeit P: Lipoprotein(a) and incident type-2 diabetes: results from the prospective Bruneck study and a meta-analysis of published literature. Cardiovasc. Diabetol. 16:38, 2017. PMID: 28320383 Journal Article
Roeseler E, Julius U, Heigl F, Spitthoever R, Heutling D, Breitenberger P, Leebmann J, Lehmacher W, Kamstrup PR, Nordestgaard BG, Maerz W, Noureen A, Schmidt K, Kronenberg F, Heibges A, Klingel R, Pro(a)LiFe-Study Group: Lipoprotein apheresis for lipoprotein(a)-associated cardiovascular disease: Prospective 5 years of follow-up and apolipoprotein(a) characterization. Arterioscler. Thromb. Vasc. Biol. 36:2019-2027, 2016. PMID: 27417585 Journal Article
Vongpromek R, Bos S, Ten Kate GJ, Yahya R, Verhoeven AJ, de Feyter PJ, Kronenberg F, Roeters van Lennep JE, Sijbrands EJ, Mulder MT: Lipoprotein(a) levels are associated with aortic valve calcification in asymptomatic patients with familial hypercholesterolaemia. J. Intern. Med. 278:166-173, 2015. PMID: 25487646 Journal Article
Willeit P, Kiechl S, Kronenberg F, Witztum JL, Santer P, Mayr M, Xu Q, Mayr A, Willeit J, Tsimikas S: Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J. Am. Coll. Cardiol. 64:851-860, 2014. PMID: 25169167 Journal Article
Frischmann ME, Ikewaki K, Trenkwalder E, Lamina C, Dieplinger B, Soufi M, Schweer H, Schaefer JR, König P, Kronenberg F, Dieplinger H: In vivo stable-isotope kinetic study suggests intracellular assembly of lipoprotein(a). Atherosclerosis 225:322-327, 2012. PMID: 23099120 Journal Article
Wassel CL, Lamina C, Nambi V, Coassin S, Mukamal KJ, Ganesh SK, Jacobs Jr DR, Franceschini N, Papanicolaou GJ, Gibson Q, Yanek LR, van der Harst P, Ferguson JF, Crawford DC, Waite LL, Allison MA, Criqui MH, McDermott MM, Mehra R, Cupples LA, Hwang SJ, Redline S, Kaplan RC, Heiss G, Rotter JI, Boerwinkle E, Taylor HA, Eraso LH, Haun M, Li M, Meisinger C, O’Connell JR, Shuldiner AR, Tybjærg-Hansen A, Frikke-Schmidt R, Kollerits B, Rantner B, Dieplinger B, Stadler M, Mueller T, Haltmayer M, Klein-Weigel P, Summerer M, Wichmann HE, Asselbergs FW, Navis G, Mateo Leach I, Brown-Gentry K, Goodloe R, Assimes TL, Becker DM, Cooke JP, Absher DM, Olin JW, Mitchell BD, Reilly MP, Mohler 3rd ER, North KE, Reiner AP, Kronenberg F, Murabito JM: Genetic determinants of the ankle-brachial index: a meta-analysis of a cardiovascular candidate gene 50K SNP panel in the candidate gene association resource (CARe) consortium. Atherosclerosis 222:138-147, 2012. PMID: 22361517 Journal Article
Brandstätter A, Lingenhel A, Zwiauer K, Strobl W, Kronenberg F: Decrease of Lp(a) during weight reduction in obese children is modified by the apo(a) kringle-IV copy number variation. Int. J. Obes. (Lond) 33:1136-1142, 2009. PMID: 19636317 Journal Article
Kronenberg F, Ikewaki K, Schaefer JR, König P, Dieplinger H: Kinetic studies of atherogenic lipoproteins in hemodialysis patients: do they tell us more about their pathology?. Semin. Dial. 20:554-560, 2007. PMID: 17991204 Review
Kiechl S, Willeit J, Mayr M, Viehweider B, Oberhollenzer M, Kronenberg F, Wiedermann CJ, Oberthaler S, Xu Q, Witztum JL, Tsimikas S: Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: prospective results from the Bruneck study. Arterioscler. Thromb. Vasc. Biol. 27:1788-1795, 2007. PMID: 17541022 Journal Article
Dieplinger B, Lingenhel A, Baumgartner N, Poelz W, Dieplinger H, Haltmayer M, Kronenberg F, Mueller T: Increased serum lipoprotein(a) concentrations and low molecular weight phenotypes of apolipoprotein(a) are associated with symptomatic peripheral arterial disease. Clin. Chem. 53:1298-1305, 2007. PMID: 17525104 Journal Article
Tsimikas S, Kiechl S, Willeit J, Mayr M, Miller ER, Kronenberg F, Xu Q, Bergmark C, Weger S, Oberhollenzer F, Witztum JL: Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J. Am. Coll. Cardiol. 47:2219-2228, 2006. PMID: 16750687 Journal Article
Kronenberg F, Lingenhel A, Lhotta K, Rantner B, Kronenberg MF, König P, Thiery J, Koch M, von Eckardstein A, Dieplinger H: Lipoprotein(a)- and low-density lipoprotein-derived cholesterol in nephrotic syndrome: Impact on lipid-lowering therapy?. Kidney Int. 66:348-354, 2004. PMID: 15200443 Journal Article







